See P-15 Osteogenic Cell Binding Peptide in action.
The power of P-15 Peptide is only found in Cerapedics spinal bone grafts.1,2
IN THE HUMAN BODY
P-15 Peptide is a naturally occurring 15-amino acid sequence found in type-1 collagen and serves as a powerful cell attachment factor. 3-6

AT CERAPEDICS
Pharmaceutically manufactured P-15 Peptide is bound onto calcium phosphate particles, creating a P-15 enhanced scaffold that provides an abundance of attachment sites for osteogenic cells.4-7
P-15 Peptide provides a distinct and proven mechanism of action, to attach and activate osteogenic cells to accelerate new bone formation.4-6,8,9
ATTACH

Osteogenic cells attach to P-15 via specific surface receptors through high-affinity interactions on the cell surface, enhancing cell retention, spreading, and survival.3-6
ACTIVATE
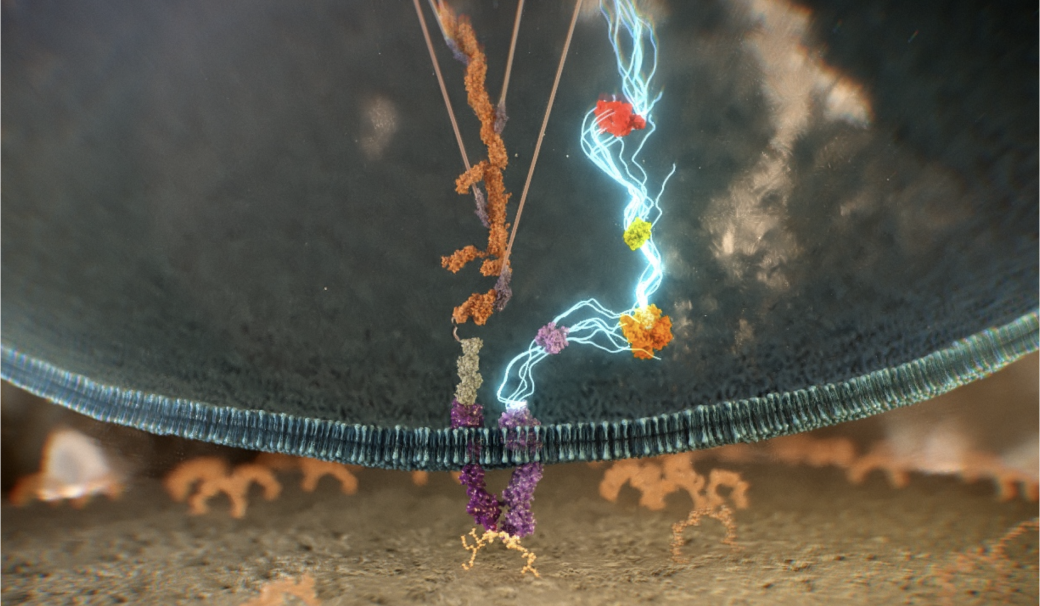
Once attached, cellular pathways are activated that enhance osteogenic activity by releasing growth factors and other important signaling molecules.3-5,10
ACCELERATE

Upregulation of growth factors recruit additional osteogenic cells, creating a cyclical attachment and activation process that accelerates new bone formation.4-6,8-12
The proof behind P-15 peptide.
Clinical evidence sets bone graft products apart. Decades of rigorous research support how P-15 Peptide transforms the way bone grafts work.

Research with us.
Be part of advancing bone repair by participating in ongoing research.
Get involvedReferences:
- PearlMatrix US Instructions for Use. Cerapedics.
- i-FACTOR US Instructions for Use. Cerapedics.
- Hanks T, et al. Biomaterials. 2004;25:4832-4836.
- Nguyen H, et al. Biochem Biophys Res Commun. 2003;311(1):179-186.
- Yang XB, et al. Tissue Eng. 2004;10(7-8):1148-1159.
- Liu Q, et al. J Orthop Res. 2012;10:1526.
- Cerapedics. Data on file.
- Thorwarth M, et al. Biomaterials. 2005;26(28):5648-5657.
- Lindley EM, et al. J Biomed Mater Res B Appl Biomater. 2010;94(2):463-468.
- Emecen P, et al. Acta Odontol Scand. 2009;67(2):65-73.
- Lind M, et al. Bone. 1996;18(1):53-57.
- Lee DH, et al. Tissue Eng. 2006;12(6):1577-1586.
